Silexion Therapeutics Reports Positive Preclinical Data Demonstrating SIL204’s Reach and Activity in Major Pancreatic Cancer Metastatic Sites Following Systemic Administration
Silexion Therapeutics (NASDAQ:SLXN) announced positive preclinical data for its pancreatic cancer drug SIL204. The study demonstrated that subcutaneously administered SIL204 successfully reached major pancreatic cancer metastatic sites and showed anti-tumor activity.
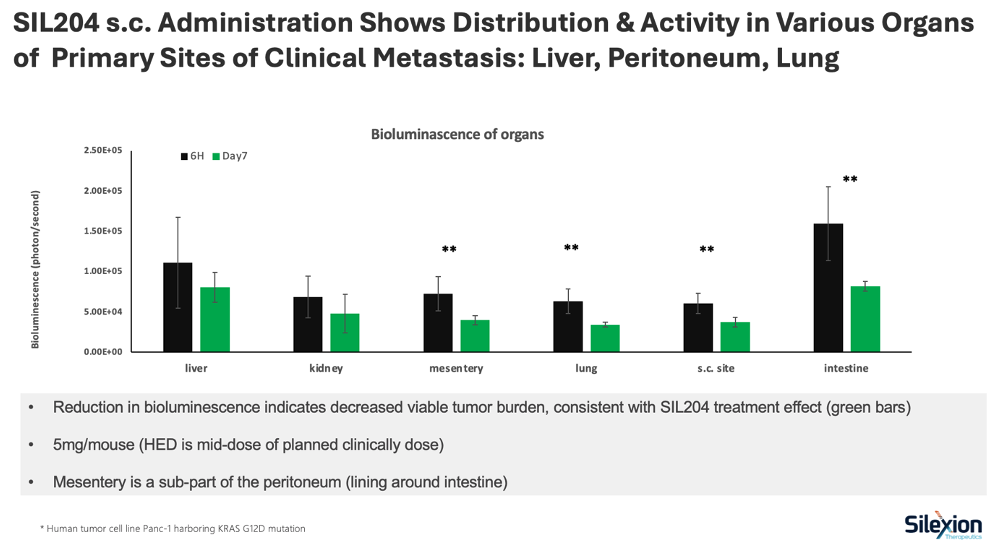
Key findings include successful drug distribution to liver, peritoneum, and lung following a single subcutaneous injection at 5mg/mouse, with statistically significant reductions (p<0.01) in tumor burden observed in the peritoneum, lung, and intestine. The results validate the systemic component of Silexion's dual-route administration strategy.
The company remains on track to initiate Phase 2/3 clinical trials in H1 2026, with regulatory submissions planned for Q4 2025 and Q1 2026.
Silexion Therapeutics (NASDAQ:SLXN) ha annunciato dati preclinici positivi per il suo farmaco per il cancro pancreatico SIL204. Lo studio ha dimostrato che la somministrazione sottocutanea di SIL204 ha raggiunto con successo i principali siti di metastasi del pancreas e ha mostrato attività antitumorale.
I risultati principali includono la distribuzione efficace del farmaco a fegato, peritoneo e polmoni dopo una singola iniezione sottocutanea da 5 mg/topo, con riduzioni del carico tumorale statisticamente significative (p<0.01) osservate in peritoneo, polmoni e intestino. I risultati confermano la componente sistemica della strategia di somministrazione a doppia via di Silexion.
L'azienda resta in linea per avviare le prove cliniche di Fase 2/3 nella prima metà del 2026, con sottomissioni regolatorie previste per il Q4 2025 e il Q1 2026.
Silexion Therapeutics (NASDAQ:SLXN) anunció datos preclínicos positivos para su fármaco contra el cáncer de páncreas SIL204. El estudio demostró que la administración subcutánea de SIL204 alcanzó con éxito los principales sitios de metástasis pancreáticas y mostró actividad antitumoral.
Los hallazgos clave incluyen la distribución eficaz del fármaco a hígado, peritoneo y pulmón tras una única inyección subcutánea de 5 mg/ratón, con reducciones del tumor estadísticamente significativas (p<0.01) observadas en peritoneo, pulmón e intestino. Los resultados validan el componente sistémico de la estrategia de administración de doble vía de Silexion.
La compañía continúa en camino para iniciar ensayos clínicos de Fase 2/3 en la primera mitad de 2026, con presentaciones regulatorias planificadas para el Q4 2025 y el Q1 2026.
Silexion Therapeutics (NASDAQ:SLXN)�� 췌장�� 치료�� SIL204�� 대�� 전임�� 긍정�� 데이터를 발표했습니다. 연구�� 피하 투여�� SIL204가 주요 췌장�� 전이 부위에 성공적으�� 도달했으�� 항종�� 활성�� 보였음을 입증했습니다.
주요 결과로는 1�� 피하 주사(5mg/마우��) �� ��, 복막, 폐로 약물�� 성공적으�� 분포했으�� 복막, �� �� 장에�� 종양 부담이 통계적으�� 유의미하�� 감소(p<0.01)�� 점이 포함됩니��. �� 결과�� Silexion�� 이중 투여 경로 전략�� 전신�� 요소�� 검증합니다.
��사��� 2026�� 상반���� 2/3�� 임상시험 시작�� 목표�� 하며, 규제 당국 제출은 2025�� 4분기와 2026�� 1분기�� 계획되어 있습니다.
Silexion Therapeutics (NASDAQ:SLXN) a annoncé des résultats précliniques positifs pour son médicament contre le cancer du pancréas SIL204. L'étude a montré que l'administration sous-cutanée de SIL204 atteignait avec succès les principaux sites de métastases pancréatiques et présentait une activité antitumorale.
Les résultats clés incluent une distribution efficace du médicament au foie, au péritoine et aux poumons après une injection sous‑cutanée unique de 5 mg/souris, avec des réductions du fardeau tumoral statistiquement significatives (p<0.01) observées au niveau du péritoine, des poumons et de l'intestin. Ces résultats valident la composante systémique de la stratégie d'administration à double voie de Silexion.
La société reste en bonne voie pour initier des essais cliniques de phase 2/3 au premier semestre 2026, avec des soumissions réglementaires prévues pour le T4 2025 et le T1 2026.
Silexion Therapeutics (NASDAQ:SLXN) gab positive präklinische Daten für sein Pankreaskrebs-Medikament SIL204 bekannt. Die Studie zeigte, dass subkutan verabreichtes SIL204 die wichtigsten Metastasierungsstellen von Bauchspeicheldrüsenkrebs erfolgreich erreichte und antitumorale Aktivität zeigte.
Zentrale Befunde sind die erfolgreiche Verteilung des Wirkstoffs in Leber, Peritoneum und Lunge nach einer einmaligen subkutanen Injektion von 5 mg/Maus, wobei in Peritoneum, Lunge und Darm statistisch signifikante Reduktionen (p<0.01) der Tumorlast beobachtet wurden. Die Ergebnisse bestätigen die systemische Komponente der dualen Applikationsstrategie von Silexion.
Das Unternehmen bleibt im Plan, Phase-2/3-Studien in H1 2026 zu starten, mit behördlichen Einreichungen, die für Q4 2025 und Q1 2026 vorgesehen sind.
- Successful drug distribution to all major metastatic sites at clinically relevant doses
- Statistically significant reductions in tumor burden across multiple sites
- Results validate dual-route administration strategy for both primary and metastatic tumors
- On track for Phase 2/3 trial initiation in H1 2026
- Still in preclinical stage, requiring successful completion of clinical trials
- Regulatory approvals pending for Q4 2025 and Q1 2026 submissions
Insights
Silexion's preclinical data shows SIL204 effectively targets pancreatic cancer metastases via subcutaneous delivery, supporting their dual-route treatment strategy.
The new preclinical data for SIL204 represents a notable development in pancreatic cancer treatment. Using a bioluminescent imaging mouse model with human pancreatic cancer cells harboring the KRAS G12D mutation, Silexion has demonstrated that subcutaneously administered SIL204 can successfully distribute to the liver, peritoneum, and lung - the primary sites where pancreatic cancer metastasizes.
This is particularly significant because over 80% of pancreatic cancer mortality stems from metastatic disease, and more than 40% of initially resectable patients experience recurrence within 12 months, predominantly as distant metastases. The ability to reach these sites systemically addresses a fundamental challenge in pancreatic cancer management.
The study showed statistically significant reductions (p<0.01) in tumor burden in the peritoneum, lung, and intestine by day 7, with measurable reductions also observed in the liver - the most common metastatic site. Importantly, these results were achieved at a 5mg/mouse dose, which represents a mid-range human equivalent dose planned for clinical trials, suggesting good translatability to human applications.
Their dual-route administration strategy - combining intratumoral delivery for primary tumors with systemic administration for metastatic disease - offers a more comprehensive approach than many current treatments. This could potentially address both local disease control and the metastatic spread that ultimately drives mortality in pancreatic cancer patients.
With regulatory submissions planned for late 2025/early 2026 and Phase 2/3 trials set to begin in H1 2026, Silexion is advancing this RNAi therapeutic approach targeting KRAS-driven cancers, which remain among the most challenging oncogenic drivers to therapeutically address.
New Positive Preclinical Data Shows Succesful Drug Distribution to Liver, Peritoneum, and Lung with Measurable Reductions in Tumor Burden at Clinically Relevant Doses
Results Provide Further Validation of the Systemic Component of a Dual-Route Administration Strategy Enabeling Potential Targeting of Both Primary Tumors and Metastatic Disease
Silexion Remains on Track for Phase 2/3 Trial Initiation in H1 2026 Following Planned Q4 2025 and Q1 2026 Regulatory Submissions
GRAND CAYMAN, Cayman Islands, Sept. 11, 2025 (GLOBE NEWSWIRE) -- Silexion Therapeutics Corp. (NASDAQ: SLXN), a clinical-stage biotechnology company pioneering RNA interference (RNAi) therapies for KRAS-driven cancers, today announced new preclinical data demonstrating that subcutaneously administered SIL204 successfully reaches all primary sites of pancreatic cancer metastasis and shows anti-tumor activity.
The study evaluated SIL204's biodistribution and therapeutic activity following subcutaneous administration in a metastatic pancreatic cancer mouse model using bioluminescent imaging. Results confirmed that SIL204 distributed to key organs where pancreatic cancer commonly spreads, with measurable reductions in tumor burden observed across multiple sites.
The ability to reach metastatic sites is particularly important given that over
"These findings provide additional validation for a critical component of our dual-route administration strategy - the ability of subcutaneously delivered SIL204 to reach metastatic sites throughout the body," said Mitchell Shirvan, Ph.D., Chief Scientific Officer of Silexion. "Demonstrating drug distribution to the liver, peritoneum, and lung, which represent the primary sites of metastatic pancreatic cancer spread, supports our approach of combining intratumoral delivery for primary tumors with systemic administration for disseminated disease."
Key Study Findings:
- SIL204 successfully distributed to all major metastatic sites following a single subcutaneous injection at 5mg/mouse (mid-range human equivalent dose for planned clinical trials)
- Reductions in bioluminescent signal, indicating decreased tumor burden, were observed at day 7 across all evaluated organs
- Statistically significant reductions (p<0.01) were achieved in the peritoneum (mesentery), lung, and intestine
- The liver, the most common site of pancreatic cancer metastasis, showed measurable reduction in tumor burden
- Studies utilized human pancreatic cancer cells (Panc-1) harboring the KRAS G12D mutation
- The use of human equivalent dosing demonstrates that these results were achieved at drug concentrations directly relevant to planned clinical use, providing important validation for the transition from preclinical to human studies
"This data addresses a fundamental challenge in pancreatic cancer treatment - reaching micrometastases that have spread beyond the primary tumor," added Ilan Hadar, Chairman and CEO of Silexion. "Combined with our previously reported intratumoral efficacy data, we now have evidence supporting both components of our treatment approach designed to comprehensively address this aggressive disease."
The Company is also conducting expanded tissue culture studies across multiple cancer types and KRAS mutations to further characterize SIL204's pan-KRAS potential, with results expected in the near future.
Silexion remains on track to initiate Phase 2/3 clinical trials evaluating its dual-route administration approach in the first half of 2026, with regulatory submissions planned for Q4 2025 and Q1 2026.
About Silexion Therapeutics
Silexion Therapeutics is a pioneering clinical stage, oncology-focused biotechnology company dedicated to the development of innovative treatments for unsatisfactorily treated solid tumor cancers which have the mutated KRAS oncogene, generally considered to be the most common oncogenic gene driver in human cancers. The Company conducted a Phase 2a clinical trial in its first-generation product which showed a positive trend in comparison to the control of chemotherapy alone. Silexion is committed to pushing the boundaries of therapeutic advancements in the field of oncology, and further developing its lead product candidate for locally advanced pancreatic cancer. For more information please visit: https://silexion.com
Notice Regarding Forward-Looking Statements:
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion's business strategy, preclinical and clinical development plans, timeline regulatory submissions and Phase 2/3 trial initiation, and expectations regarding SIL204's therapeutic potential, are forward-looking statements. These forward-looking statements are generally identified by terminology such as "may", "should", "could", "might", "plan", "possible", "project", "strive", "budget", "forecast", "expect", "intend", "will", "estimate", "anticipate", "believe", "predict", "potential" or "continue", or the negatives of these terms or variations of them or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied in those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion's ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion's strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion's future capital requirements and sources and uses of cash, including its ability to obtain additional capital; (vi) Silexion's ability to maintain its Nasdaq listing; and (vii) other risks and uncertainties set forth in the documents filed with the SEC by the Company, including the Company's Annual Report on Form 10-K for the year ended December 31, 2024. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact:
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
Capital Markets & IR Contact:
Arx Capital Markets
North American Equities Desk









