Silexion Therapeutics Announces Positive Results in Preclinical Study Demonstrating Significant SIL204 Efficacy in Human Lung Cancer Cell Lines
Silexion Therapeutics (NASDAQ: SLXN) has announced positive preclinical results for its SIL204 drug candidate in human lung cancer cell lines. The study demonstrated significant dose-dependent inhibition in lung cancer cells with KRAS G12D mutations, validating the company's lipid-conjugated delivery system.
The company is preparing for a Phase 2/3 clinical trial in Q2 2026 to investigate SIL204 for KRAS-driven solid tumor cancers, utilizing both intratumoral and systemic delivery approaches. Silexion is also conducting additional studies on a previously untested KRAS mutation to potentially establish SIL204 as a pan-KRAS treatment.
The target market is substantial, with KRAS mutations present in 90% of pancreatic cancers, 45% of colorectal cancers, and 30% of lung cancers, representing a combined global treatment market exceeding $30 billion annually.
Silexion Therapeutics (NASDAQ: SLXN) ha annunciato risultati preclinici positivi per il suo candidato farmaco SIL204 in linee cellulari di cancro polmonare umano. Lo studio ha dimostrato una significativa inibizione dose-dipendente nelle cellule di cancro polmonare con mutazioni KRAS G12D, confermando l'efficacia del sistema di somministrazione coniugato ai lipidi sviluppato dall'azienda.
L'azienda si sta preparando per una fase 2/3 di sperimentazione clinica nel secondo trimestre del 2026 per studiare SIL204 nei tumori solidi guidati da KRAS, utilizzando sia approcci di somministrazione intratumorale che sistemica. Silexion sta inoltre conducendo ulteriori studi su una mutazione KRAS finora non testata per potenzialmente affermare SIL204 come trattamento pan-KRAS.
Il mercato target è significativo, con mutazioni KRAS presenti nel 90% dei tumori pancreatici, 45% dei tumori del colon-retto e 30% dei tumori polmonari, rappresentando un mercato globale combinato per il trattamento che supera i 30 miliardi di dollari all'anno.
Silexion Therapeutics (NASDAQ: SLXN) ha anunciado resultados preclínicos positivos para su candidato a fármaco SIL204 en líneas celulares de cáncer de pulmón humano. El estudio mostró una inhibición significativa dependiente de la dosis en células de cáncer de pulmón con mutaciones KRAS G12D, validando el sistema de administración conjugado a lípidos de la compañía.
La empresa se está preparando para un ensayo clínico de fase 2/3 en el segundo trimestre de 2026 para investigar SIL204 en tumores sólidos impulsados por KRAS, utilizando enfoques de administración intratumoral y sistémica. Silexion también está realizando estudios adicionales sobre una mutación KRAS no probada previamente para establecer potencialmente SIL204 como un tratamiento pan-KRAS.
El mercado objetivo es considerable, con mutaciones KRAS presentes en el 90% de los cánceres de páncreas, 45% de los cánceres colorrectales y 30% de los cánceres de pulmón, representando un mercado global combinado para tratamiento que supera los 30 mil millones de dólares anuales.
Silexion Therapeutics (NASDAQ: SLXN)가 인간 폐암 세포주에�� SIL204 약물 후보�� 긍정적인 전임�� 결과�� 발표했습니다. 연구�� KRAS G12D 돌연변이를 가�� 폐암 세포에서 용량 의존적인 유의미한 억제 효과�� 보여 회사�� 지�� 결합 전달 시스템을 검증했습니��.
회사�� 2026�� 2분기�� SIL204�� KRAS 유발 고형 종양�� 적용하기 위한 2/3�� 임상시험�� 준�� 중이��, 종양 �� �� 전신 투여 방식�� 모두 활용�� 예정입니��. 또한, Silexion은 이전�� 시험되지 않은 KRAS 돌연변이에 대�� 추가 연구�� 진행하여 SIL204�� ��-KRAS 치료제로 확립�� 가능성�� 모색하고 있습니다.
목표 시장은 크며, KRAS 돌연변이는 췌장암의 90%, 대장암�� 45%, 폐암�� 30%에서 발견되어 연간 300�� 달러가 넘는 �� 세계 치료 시장�� 형성합니��.
Silexion Therapeutics (NASDAQ : SLXN) a annoncé des résultats précliniques positifs pour son candidat médicament SIL204 dans des lignées cellulaires de cancer du poumon humain. L'étude a démontré une inhibition significative dépendante de la dose dans les cellules de cancer du poumon portant des mutations KRAS G12D, validant le système de délivrance conjugué aux lipides de la société.
L'entreprise se prépare à un essai clinique de phase 2/3 au deuxième trimestre 2026 pour étudier SIL204 dans les tumeurs solides à mutation KRAS, utilisant à la fois des approches d'administration intratumorale et systémique. Silexion mène également des études supplémentaires sur une mutation KRAS encore non testée afin de potentiellement établir SIL204 comme traitement pan-KRAS.
Le marché cible est important, avec des mutations KRAS présentes dans 90 % des cancers du pancréas, 45 % des cancers colorectaux et 30 % des cancers du poumon, représentant un marché mondial combiné de traitement dépassant 30 milliards de dollars par an.
Silexion Therapeutics (NASDAQ: SLXN) hat positive präklinische Ergebnisse für seinen Wirkstoffkandidaten SIL204 in humanen Lungenkrebszelllinien bekannt gegeben. Die Studie zeigte eine signifikante dosisabhängige Hemmung in Lungenkrebszellen mit KRAS G12D-Mutationen und bestätigte damit das lipidgebundene Liefersystem des Unternehmens.
Das Unternehmen bereitet eine Phase-2/3-Studie im zweiten Quartal 2026 vor, um SIL204 bei KRAS-getriebenen soliden Tumoren zu untersuchen, wobei sowohl intratumorale als auch systemische Verabreichungswege genutzt werden. Silexion führt zudem weitere Studien zu einer bisher ungetesteten KRAS-Mutation durch, um SIL204 möglicherweise als pan-KRAS-Therapie zu etablieren.
Der Zielmarkt ist beträchtlich, da KRAS-Mutationen bei 90 % der Bauchspeicheldrüsenkrebsfälle, 45 % der kolorektalen Krebsfälle und 30 % der Lungenkrebsfälle vorkommen und einen weltweiten Behandlungsmarkt von über 30 Milliarden US-Dollar jährlich darstellen.
- Significant dose-dependent inhibition demonstrated in lung cancer cells with KRAS G12D mutations
- Successful validation of lipid-conjugated delivery system for enhanced tumor cell penetration
- Phase 2/3 clinical trial planned for Q2 2026
- Large addressable market exceeding $30 billion annually across three cancer types
- Potential for broad application across multiple KRAS-driven cancer types
- Still in preclinical stage with significant development timeline ahead
- Phase 2/3 trial not starting until Q2 2026
- Results from new KRAS mutation study still pending
- Faces competition in crowded oncology market
Insights
Silexion's SIL204 shows promising preclinical results against KRAS-mutated lung cancer, but remains years from potential market approval.
Silexion Therapeutics has reported positive preclinical data for their RNA interference (RNAi) therapy SIL204 in human lung cancer cell lines with KRAS G12D mutations. The results demonstrated significant dose-dependent inhibition, supporting their lipid-conjugated delivery system's ability to overcome a critical barrier in siRNA technology - getting the therapeutic molecules into solid tumor cells.
The significance of this development lies in the RNAi approach to KRAS mutations, which are notoriously difficult to target with conventional therapeutics. While small molecule inhibitors target already-produced mutant KRAS proteins, SIL204 aims to silence KRAS production at the genetic level. This approach could potentially address multiple KRAS variants across different cancer types.
These results build on the company's previous findings in pancreatic and colorectal cancer models. Silexion is also conducting studies on a previously untested KRAS mutation to potentially position SIL204 as a pan-KRAS treatment - addressing a mutation present in approximately 90% of pancreatic cancers, 45% of colorectal cancers, and 30% of lung cancers.
However, investors should note that SIL204 remains in early development stages. The company plans to initiate a Phase 2/3 clinical trial in Q2 2026, meaning that even with positive outcomes, commercial availability would still be several years away. The substantial market opportunity cited (over $30 billion annually across the three cancer indications) represents the potential addressable market, not projected revenue.
While these preclinical results are encouraging, they represent very early evidence of efficacy. The transition from cell line studies to human trials involves significant scientific and regulatory hurdles, with many promising preclinical candidates ultimately failing to demonstrate comparable efficacy and safety in human subjects.
Company’s New Groundbreaking Preclinical Data in NSCLC Models Provides Further Validation for SIL204's Innovative Delivery System; Company is Currently Conducting Additional Studies into New and Previously Untested KRAS Mutation with Results expected in the Near Future
Grand Cayman, Cayman Islands, July 09, 2025 (GLOBE NEWSWIRE) -- Silexion Therapeutics Corp. (NASDAQ: SLXN) ("Silexion" or the "Company"), a clinical-stage biotechnology company pioneering RNA interference (RNAi) therapies for KRAS-driven cancers, today announced new positive preclinical data demonstrating SIL204's significant efficacy in human lung cancer cell lines.
The Company is also pleased to announce that it is in the process of conducting a new study examining the efficacy of SIL204 on a new previously untested KRAS mutation, the results of which it plans to release shortly. If positive, such results could further help establish SIL204 as a potential pan-KRAS treatment.
In parallel, Silexion's dual-route administration strategy, leveraging both intratumoral and systemic delivery approaches, remains on track. The Company continues to prepare for the initiation of a Phase 2/3 clinical trial in Q2 2026 to investigate SIL204 for the treatment of KRAS-driven solid tumor cancers.
Key Study Findings:
The study revealed significant dose-dependent inhibition in lung cancer cells harboring KRAS G12D mutations, with notable efficacy, highlighting SIL204's potential as a versatile therapeutic for lung cancer. The results also support Silexion’s lipid-conjugated delivery system for enhancing SIL204 drug entrance into tumor cells; overcoming a known barrier for siRNA technology and one which is critical for therapeutic efficacy in solid tumors.
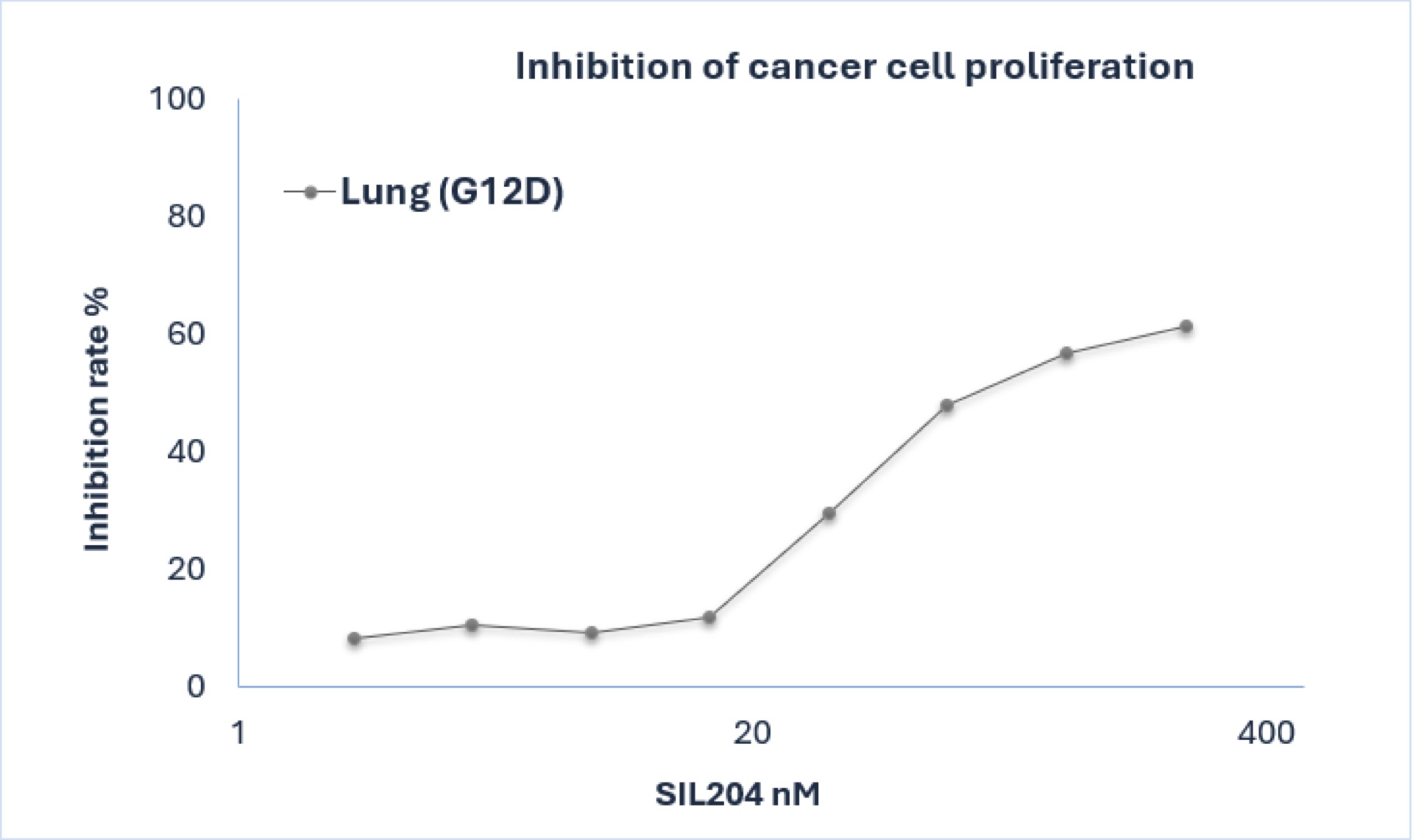
Figure: SIL204 demonstrates dose-dependent inhibition in human lung cancer cells harboring KRAS G12D mutation showing robust efficacy at higher concentrations.
"These new findings provide compelling evidence of SIL204's enhanced delivery capabilities in lung cancer models," said Ilan Hadar, Chairman and Chief Executive Officer of Silexion. "The ability of our lipid-conjugated siRNA to enter cancer cells represents a significant advantage for clinical applications, potentially overcoming a major challenge in RNAi therapeutics for solid tumors."
In May 2025, Silexion the completion of initial studies exploring SIL204's potential impact on colorectal and lung cancer, noting plans to conduct additional studies on lung cancer cell lines in the coming weeks. Today's announcement fulfills that commitment, providing further validation of SIL204's mechanism of action and broad potential across multiple KRAS-driven cancer types.
These new results provide further validation for Silexion's innovative approach to KRAS-targeting through RNA interference. Unlike small molecule inhibitors that target already-produced mutant KRAS proteins, SIL204 is designed to silence the production of oncogenic KRAS at the genetic level, preventing the production of these cancer-driving proteins at their source.
KRAS mutations are among the most common oncogenic drivers in human cancers, occurring in roughly
This announcement builds on Silexion's previously reported findings demonstrating SIL204's efficacy in both pancreatic and colorectal cancer models. Together, these results underscore the potential of SIL204 as a pan-KRAS treatment for multiple challenging cancer types characterized by historically difficult-to-drug KRAS mutations.
About Silexion Therapeutics
Silexion Therapeutics is a pioneering clinical-stage, oncology-focused biotechnology company developing innovative RNA interference (RNAi) therapies to treat solid tumors driven by KRAS mutations, the most common oncogenic driver in human cancers. The Company’s first-generation product, LODER��, has shown promising results in a Phase 2 trial for non-resectable pancreatic cancer. Silexion is also advancing its next-generation siRNA candidate, SIL204, designed to target a broader range of KRAS mutations and showing significant potential in preclinical studies. The Company remains committed to pushing the boundaries of therapeutic innovation in oncology, with a focus on improving outcomes for patients with difficult-to-treat cancers. For more information please visit:
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion's business strategy, ongoing preclinical studies evaluating SIL204 in colorectal and lung cancer applications, potential expansion of development strategy, and the therapeutic potential of SIL204 across multiple cancer types, are forward-looking statements. These forward-looking statements are generally identified by terminology such as "may", "should", "could", "might", "plan", "possible", "project", "strive", "budget", "forecast", "expect", "intend", "will", "estimate", "anticipate", "believe", "predict", "potential" or "continue", or the negatives of these terms or variations of them, or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied by those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion's ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion's strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion's future capital requirements and sources and uses of cash, including its ability to obtain additional capital; (vi) Silexion’s ability to maintain its Nasdaq listing; and (vii) other risks and uncertainties set forth in the documents filed or to be filed with the SEC by the Company, including the Company's Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 18, 2025. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
Capital Markets & IR Contact
Arx Capital Markets
North American Equities Desk
[1] (i) ; (ii) ; (iii)








