Annovis Completes Full Patent Transfer to Crystal Buntanetap
Annovis Bio (NYSE: ANVS) has successfully completed the transfer of all patents to cover both the original semi-crystalline and new crystalline forms of buntanetap, its drug candidate for neurodegenerative diseases. The comprehensive IP portfolio now includes 13 patent families with protection extending to 2046.
The new crystalline form demonstrates improved solid-state stability and comparable pharmacokinetic properties to the original form, with bioequivalence confirmed through animal and human studies. The FDA has already approved the use of the crystalline form in the company's ongoing Phase 3 clinical trial for early Alzheimer's disease, which is currently active with over 75 US sites.
Annovis Bio (NYSE: ANVS) ha completato con successo il trasferimento di tutti i brevetti che coprono sia la forma semi-cristallina originale sia la nuova forma cristallina di buntanetap, il suo candidato farmaco per le malattie neurodegenerative. Il portafoglio di proprietà intellettuale ora comprende 13 famiglie di brevetti con protezione estesa fino al 2046.
La nuova forma cristallina mostra una stabilità allo stato solido migliorata e proprietà farmacocinetiche comparabili alla forma originale, con bioequivalenza confermata tramite studi su animali e umani. La FDA ha già approvato l'uso della forma cristallina nel trial clinico di Fase 3 per l'Alzheimer precoce attualmente in corso, che coinvolge oltre 75 siti negli Stati Uniti.
Annovis Bio (NYSE: ANVS) ha completado con éxito la transferencia de todas las patentes que cubren tanto la forma semicristalina original como la nueva forma cristalina de buntanetap, su candidato a medicamento para enfermedades neurodegenerativas. La cartera integral de propiedad intelectual ahora incluye 13 familias de patentes con protección extendida hasta 2046.
La nueva forma cristalina demuestra una estabilidad en estado sólido mejorada y propiedades farmacocinéticas comparables a la forma original, con bioequivalencia confirmada mediante estudios en animales y humanos. La FDA ya ha aprobado el uso de la forma cristalina en el ensayo clínico de fase 3 para la enfermedad de Alzheimer temprana que la compañía está llevando a cabo, con más de 75 sitios activos en EE.UU.
Annovis Bio (NYSE: ANVS)�� 신경퇴행�� 질환 치료 후보 약물�� buntanetap�� 원래 반결정질 �� 새로�� 결정�� 형태�� 모두 포함하는 특허 이전�� 성공적으�� 완료했습니다. 포괄적인 지�� 재산�� 포트폴리오는 이제 13�� 특허���� 포함하며 보호 기간은 2046���까지 연장됩니��.
새로�� 결정�� 형태�� 고체 상태 안정성이 향상되었으며 원래 형태와 비교�� 약동학적 특성�� 유사하며, 동물 �� 인간 연구�� 통해 생물학적 동등성이 확인되었습니��. FDA�� 이미 회사�� 진행 중인 초기 알츠하이머병 3�� 임상시험에서 결정�� 형태 사용�� 승인했으��, 현재 미국 �� 75�� 이상�� 사이트에�� 활발�� 진행 중입니다.
Annovis Bio (NYSE : ANVS) a réussi le transfert de tous les brevets couvrant à la fois la forme semi-cristalline originale et la nouvelle forme cristalline de buntanetap, son candidat médicament pour les maladies neurodégénératives. Le portefeuille complet de propriété intellectuelle comprend désormais 13 familles de brevets avec une protection étendue jusqu'en 2046.
La nouvelle forme cristalline présente une stabilité à l'état solide améliorée et des propriétés pharmacocinétiques comparables à la forme originale, avec une bioéquivalence confirmée par des études animales et humaines. La FDA a déjà approuvé l'utilisation de la forme cristalline dans l'essai clinique de phase 3 en cours pour la maladie d'Alzheimer précoce, qui compte actuellement plus de 75 sites actifs aux États-Unis.
Annovis Bio (NYSE: ANVS) hat erfolgreich die Übertragung aller Patente abgeschlossen, die sowohl die ursprüngliche halb-kristalline als auch die neue kristalline Form von buntanetap, dem Wirkstoffkandidaten für neurodegenerative Erkrankungen, abdecken. Das umfassende IP-Portfolio umfasst nun 13 Patentfamilien mit Schutz bis 2046.
Die neue kristalline Form zeigt eine verbesserte Feststoffstabilität und vergleichbare pharmakokinetische Eigenschaften zur ursprünglichen Form, wobei die Bioäquivalenz durch Tier- und Humanstudien bestätigt wurde. Die FDA hat bereits die Verwendung der kristallinen Form in der laufenden Phase-3-Studie bei frühem Alzheimer genehmigt, die derzeit mit über 75 Standorten in den USA aktiv ist.
- Secured comprehensive patent protection for both drug forms until 2046
- New crystalline form shows improved stability while maintaining equivalent effectiveness
- FDA approval received for using new crystal form in Phase 3 trial
- Active Phase 3 trial with over 75 sites across US
- None.
Insights
Annovis strengthens IP position with full patent protection for both forms of buntanetap through 2046, reducing competitive threats and enhancing commercialization potential.
Annovis has secured a significant IP milestone by successfully transferring all patents to cover both the original semi-crystalline and new crystalline forms of buntanetap. The company now holds 13 patent families with international filings that provide protection until 2046 - a substantial runway that dramatically enhances the commercial potential of their neurodegenerative disease candidate.
The comprehensive patent portfolio covers multiple critical aspects: composition of matter, mechanism of action, various therapeutic indications, and combination therapies. This layered approach creates a robust defensive moat around buntanetap that would be challenging for competitors to circumvent.
What's particularly notable is the strategic development of the new crystalline form, which offers improved solid-state stability while maintaining bioequivalent pharmacokinetic properties to the original formulation. This crystalline variation represents both a scientific advancement and a shrewd IP strategy that effectively extends protection and creates redundancy in their patent estate.
The FDA's approval to use the new crystalline form in their ongoing Phase 3 Alzheimer's trial (NCT06709014) validates the interchangeability of the forms from a regulatory perspective. This removes potential regulatory hurdles that could have arisen from switching formulations mid-development.
The ability to protect combination therapies with GLP-1 agonists, PDE5 inhibitors, and statins is particularly forward-thinking, as it preserves optionality for pipeline expansion while blocking potential competitive combination approaches. This comprehensive IP strategy significantly de-risks the company's development pathway and enhances its partnership and acquisition attractiveness.
Annovis achieves comprehensive intellectual property (IP) protection now covering both the original semi-crystalline and new crystalline forms of buntanetap.
Both forms are covered by a total of 13 patent families.
All patents are filed internationally, securing global coverage of buntanetap.
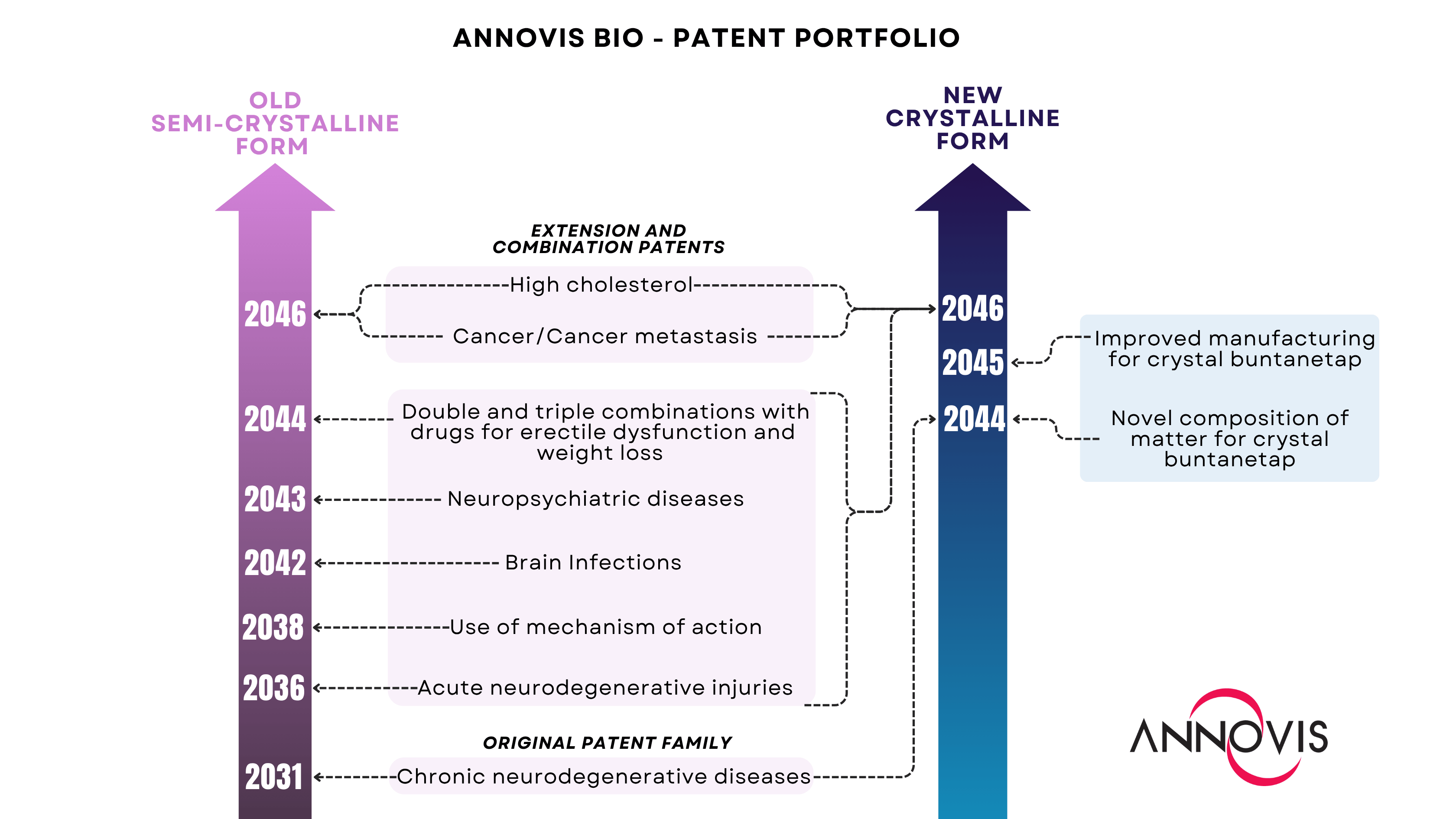
MALVERN, Pa., Aug. 07, 2025 (GLOBE NEWSWIRE) -- Annovis Bio, Inc. (NYSE: ANVS) ("Annovis" or the “Company��), a late-stage clinical drug platform company pioneering transformative therapies for neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD), today announced that all Company’s patents have been successfully transferred from the original form of buntanetap and rewritten to cover the new crystal form. Every patent family is now represented for both forms of the Company’s drug candidate, securing comprehensive protection, which extends to 2046.
Building on its foundational patent for the treatment of chronic neurodegenerative conditions, the Company has systematically expanded its coverage to include patents for the use of the original compound. Since then, Annovis has developed a robust IP portfolio which includes patents protecting the composition of matter, mechanism of action, applications of buntanetap for multiple indications, and its combination with other drugs.
Recently, the Company identified and characterized a new crystalline form of buntanetap with improved solid-state stability and comparable pharmacokinetic (PK) properties. A series of animal and human studies demonstrated the bioequivalence of both forms of buntanetap as they exhibit identical systemic exposure. These findings were presented at the Alzheimer’s Association International Conference (AAIC) 2025 in Toronto, and the poster is now available in the on the Company’s website.
Annovis previously secured protection for the composition of matter and manufacturing process of the crystalline form. However, as of this month, the Company has successfully completed the transfer of all IP inventions, extensions, and combinations from the original buntanetap to the new form, ensuring comprehensive coverage of its drug candidate on two fronts: one preserving the legacy of the semi-crystalline buntanetap (pink) and the other dedicated to the crystalline form (blue).
“This is a major milestone for our company,�� said Maria Maccecchini, Ph.D., President and CEO of Annovis. “With full IP protection now in place for both forms of buntanetap, we are well-positioned to continue its development and fully explore its therapeutic potential. Buntanetap’s unique mechanism of action allows it to suppress multiple pathologies simultaneously, and it may offer even better advantages when complemented by other drugs such as GLP-1 agonists, PDE5 inhibitors, and statins. This opens doors to possible pipeline expansions and broader clinical applications as part of our strategic growth.��
This IP milestone for crystal buntanetap complements another significant step �� last year the FDA approved the use of the new form for the pivotal Phase 3 clinical trial in early AD (), based on its safety and PK data. The trial was initiated earlier this year with participants (MMSE 21-28, pTau217+) receiving either placebo or crystal buntanetap daily for 18 months. The study is actively underway with over 75 sites secured across the US and more patients entering each week, bringing us closer to reaching our enrollment goal by day. To find more information about the study, visit our updated or the official page.
About Annovis
Headquartered in Malvern, Pennsylvania, Annovis is dedicated to addressing neurodegeneration in diseases such as AD and PD. The Company is committed to developing innovative therapies that improve patient outcomes and quality of life. For more information, visit and follow us on , , and .
Investor Alerts
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by registering for email alerts at .
Forward-Looking Statements
This press release contains forward-looking statements under the Securities Act of 1933 and the Securities Exchange Act of 1934, as amended. Actual results may differ due to various risks and uncertainties, including those outlined in the Company’s SEC filings under “Risk Factors�� in its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. The Company undertakes no obligation to update forward-looking statements except as required by law.
Contact Information:
Annovis Bio Inc.
101 Lindenwood Drive
Suite 225
Malvern, PA 19355
Investor Contact:
Alexander Morin, Ph.D.
Director, Strategic Communications
Annovis Bio
A photo accompanying this announcement is available at









